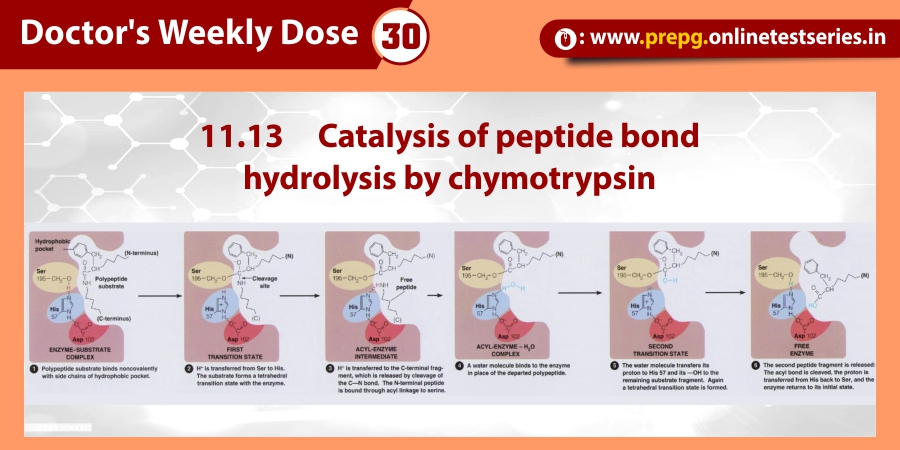
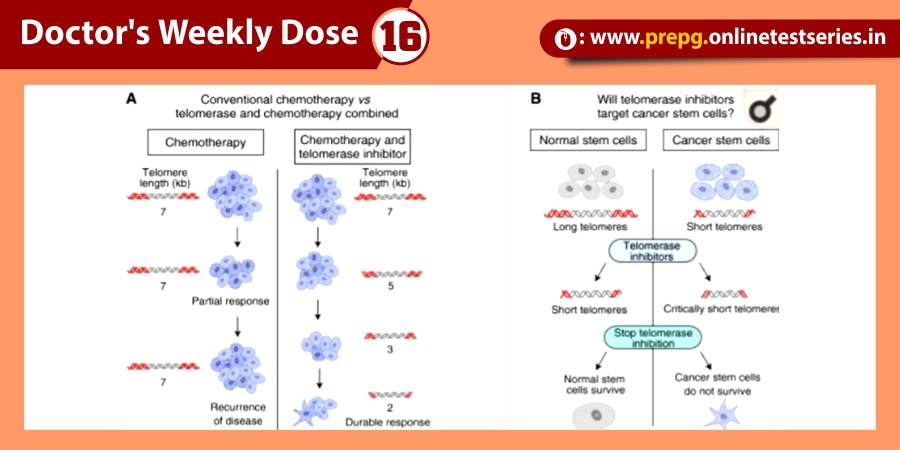
Attachment :
The gp120 attaches to the attachment receptor CD4. This attachment is reversible and important for HIV entry although not mandatory. Gp120 then binds to the entry receptor CXCR4, which is a signalling receptor. LxK kinase bound to the cytoplasmic tail of CD4 may phosphorylate CXCR4. It is unclear if the virus fuses at the cell surface or is endocytosed by the clathrin mechanism.
Virion component release in cell cytoplasm :
After virus-host membrane fusion, viral capsid is released in the cytoplasm. Vpx(HIV-2 only) is presumably transported to the nucleus where it would act to counteract host restriction factor to reverse-transcription/PIC entry.
Reverse transcription :
comment: does diploidy play a role during reverse transcription? Several studies have addressed the question, they all agree that second strand transfer is intramolecular, but the first strand transfer would be: intermolecular intramolecular or a mix of both
Transport :
The viral capsid interacts with microtubules and is actively transported to the vicinity of the cell nucleus. This transport takes 40 min ex vivo.
Uncoating and nuclear entry :
Uncoating is the process whereby the cDNA viral genome gets out of the capsid. It is a multistep process, closely linked to reverse transcription. Early uncoating may occur under certain circumstances and leads to infection abortion: CypA depletion, simian Trim5alpha binding, Mx2 antiviral protein expression or cytoplasmic CPSF6. Early uncoating may induce cytoplasmic dsDNA sensor antiviral response.
The pre-integration complex (cDNA, Integrase) entry into the nucleus occurs through various binding to nuclear pore and is dependent on the presence of vpr.
Integration :
The viral cDNA is covalently integrated into the host genome by the integrase, preferentially at transcription active places. Integrated virus genome is called the provirus.
Latency/ replication decision :
The integrated provirus can get methylated in the host genome, and this transcriptional silencing induces viral latency. Alternatively, the provirus can be transcribed enough to reach a tat expression allowing to enter in replicative phase.
Latency/ reactivation :
Cellular stress or activation can lead to nuclear signals that remove the provirus transcriptional silencing, thereby inducing the viral replication cycle.
Transcription :
Provirus transcription is under the control of tat and many host transcription factors that interacts with U3 promoter. mRNA transcripts are alternatively spliced into six mRNAs.
GRNA nuclear exit :
The unspliced genomic RNA (gRNA) cannot be exported to the cytoplasm under normal circumstance. The rev viral protein specifically unlocks gRNA export into the cytoplasm, which are both templates for GAG and POL translation, and the genetic material susceptible to bud in new virions.
Virion assembly and budding :
The genomic packaging signal (Phi) is present only in gRNA. In the monomeric gRNA, The Phi element is sequestered by intramolecular base pairing and then become exposed upon dimerization, thereby allowing specific, high-affinity binding of the gRNA dimer to the NC domain. Several GAG proteins interact with a dimer of genomic RNAs. This complex is transported by actin or microtubule to the plasma membrane where final virion assembly and budding occurs. h3. Virion maturation
Upon virion release the protease is activated and cleaves GAG and GAG-POL polyproteins into individual chains that self-assemble to form the capsid inside the virion. Mutant of Nc are reverse transcribing before budding: Nc would play a role in the trigger of processing/reverse transcription post budding. The capsid can assemble in a cylinder in some virion, those seem to have excluded virus genome form the capsid.